Juniper Publishers- Open Access Journal of Case Studies
Anal Squamous Cell Carcinoma Presenting as Perineal Sepsis: A Case Report and Literature Review
Authored by Ricardo Balanzá
Abstract
Background: Anal canal cancer is an uncommon cancer worldwide; nevertheless its incidence has risen in recent years. Squamous carcinoma is the most common histological type of anal canal cancer. The most important risk factor for squamous anal carcinoma is infection by oncogenic types of human papillomavirus. The clinical presentation for this malignancy usually includes pain, bleeding, swelling, pruritus, and discharge. The treatment goals for anal cancer include local control, ideally with sphincter preservation. The management of this malignancy has evolved from surgery to curative chemoradiation, with radical surgery as a salvage treatment.
Case report: A 53-year-old female patient presented to the emergency department with severe pain localized to the perineal area, associated with transanal bleeding, purulent drainage, fever, and the sensation of a bulge near the anus. Proctological exploration showed a left lateral swelling with erythema and a secondary opening with haemopurulent drainage, digital rectal examination revealed a left lateral mass with minimal fluctuation. A rectal exam under anesthesia was performed identifying an ulcerated mass with an associated abscess and fistulous tract. Biopsies of the indurated ulcer with rolled up borders were obtained. The pathology report revealed an invasive squamous carcinoma. We performed a CT-scan to classify the tumor as T3N0MO corresponding to NCCN clinical stage II. Serology for HIV was negative. Biopsy samples were negative for HPV infection. The patient underwent chemoradiation with Mitomycin C and Capecitabine with complete clinical response.
Keywords: Anal cancer; Anal squamous cell carcinoma; Perineal sepsis; Human papillomavirus; Chemoradiation
Introduction
Anal cancer represents approximately 1% of all gastrointestinal cancers [1]. The incidence of anal cancer around the world has risen over the last 30 years [1,2]. Anal squamous cell carcinoma (ASCC) represents between 80 and 85% of all anal canal cancers [1,2]. The oncogenic types of human papillomavirus (HPV) are etiologically linked to ASCC [1]. Risk factors for this malignancy are HPV infection, human immunodeficiency virus (HIV) infection, immune suppression in transplant recipients, smoking, receptive anal intercourse, lifetime number of sexual partners, and previous in situ or invasive cervical, vulvar or vaginal cancer [3]. Anal cancer screening is being stablished for high risk populations, such as HIV-positive individuals, in the form of anal Papanicolaou and high-resolution anoscopy [4]. ASCC can present with any combination of a mass, non-healing ulcer, bleeding, pain, pruritus, discharge, fistula, or fecal incontinence [5]. Radiation with concurrent chemotherapy is considered as the standard of care for ASCC [6]. The combination of 5-Fluorouracil (5-FU) and other cytotoxic agents as Mitomycin C (MMC) is able to achieve complete tumor regression in up to 90% of patients [5].
Case Report
A 53-year-old female patient presented to the emergency room with the chief complaint of perineal pain for the last 3 days. The pain was constant, severe, localized to the perineal area, associated to transanal bleeding, purulent drainage, fever, and the sensation of a bulge near the anus. The temperature was 38.4ºC, heart rate was 106/min, respiratory rate was 24/min, and blood pressure was 90/50 mm Hg. On physical examination the patient was dehydrated, the abdomen was flat, with normal bowel sounds, perianal inspection showed a left lateral swelling with erythema, a linear ulcer with rolled up edges suggestive of malignant disease, and a fistula opening with haemopurulent drainage, digital rectal examination revealed a left lateral mass with minimal fluctuation around approximately 40% of the anal canal. Initial blood count and blood chemistry tests were entirely normal except for an elevated leukocyte count at 18,000/mm3 with shift to the left. Past medical and surgical history were not significant. We decided to proceed to the operating room for drainage and biopsy of the mass with the suspected diagnosis of a tumor associated fistula.
A rectal exam under anesthesia was performed identifying an ulcerated mass of approximately 6x4x3cm in the left lateral position of the anal canal with an associated abscess and fistulous tract. The tumor was classified as T3NXMX at the time of surgery. Biopsies were obtained by quadrants to map the extent of the lesion, including the transition to normal tissue macroscopically, the abscess was drained and a fistulectomy was performed along with resection of the engorged hemorrhoidal tissue around the ulcer (Figure 1). A rigid sigmoidoscopy showed the absence of other lesions up to 25cm. The pathology report revealed fragments of the anal canal with focally keratinizing moderately differentiated invasive squamous carcinoma and multifocal squamous carcinoma in situ (Figure 2 & 3). Human papillomavirus was not detected in the biopsies. Human immunodeficiency virus serology was negative for types 1 and 2.
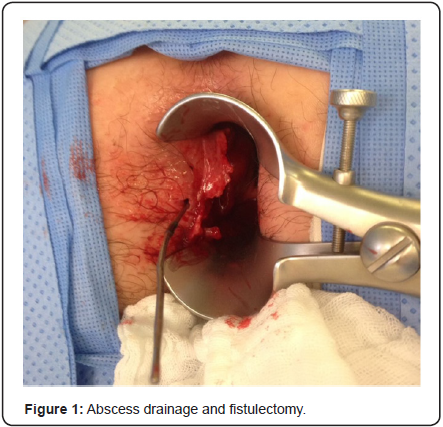
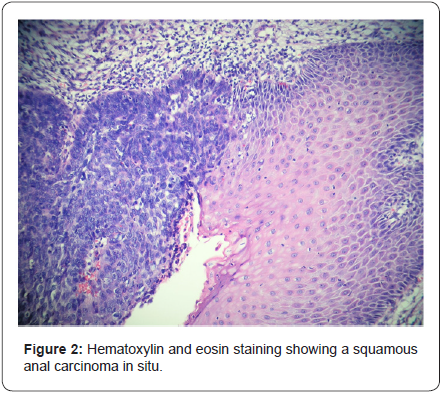
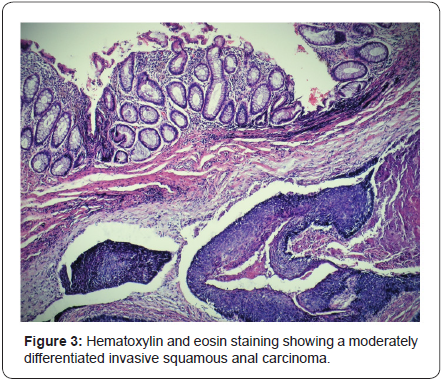
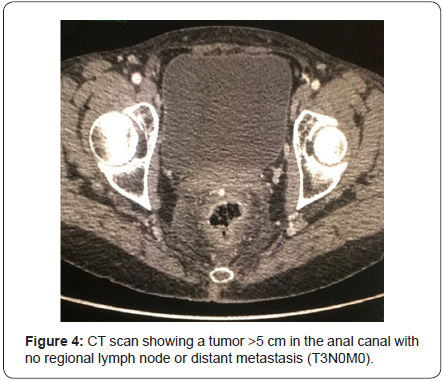
A computed tomography (CT scan) of the abdomen and pelvis was used to stage the tumor as T3N0M0 with clinical stage II (Figure 4). A Pap smear and colposcopy were performed with negative results. The patient initiated modified Nigro protocol with Mitomycin C and Capecitabine along with radiotherapy. The patient had a positron emission tomography (PET/CT) 4 months after completing chemoradiation with no evidence of persistent disease. She will have a follow-up with proctologic examination every 3 months and a PET/CT every 6 months.
Discussion
Anal cancer is a relatively uncommon cancer and accounts for 0.43% of all malignancies [4]. The incidence of this cancer has risen in the United States from 0.8 to 1.7 cases per 100,000 persons per year from 1975 to 2011 [4]. The incidence of anal cancer in England in 2010 was 1.2 per 100,000 persons per year in men and 1.6 per 100,000 persons per year in women [2]. The mean age for diagnosis in anal cancer is 60 years and only 1.1% of the cases are diagnosed in people younger than 35-years-old [7]. Anal cancer is a term that includes both anal canal and anal margin tumors [2]. The anal canal is defined as the terminal part of the large intestine extending from the anorectal junction in the upper part of the pelvic floor to the anal verge, which is the hairbearing skin around the anus [4]. The anal margin is defined as the area extending from the anal verge to 5cm circumferentially outward on the perineal skin [2].
The predominant histological type of anal canal cancer is ASCC, which constitutes between 80 and 85% of all anal canal carcinomas [1,2]. Other histological types of anal canal cancer include adenocarcinomas, which account for between 5 and 18% of anal cancers, mucinous adenocarcinomas, small cell carcinomas, undifferentiated carcinomas, carcinoid tumors, non epithelial tumors, and melanomas [2]. Factors that increase the risk of presenting ASCC include HPV infection, anal intercourse, high lifetime number of sexual partners, HIV infection, immune suppression in transplant recipients, use of immune suppressants such as long-term corticosteroids, a history of other HPV-related cancers, autoimmune disorders, social deprivation, and cigarette smoking [5]. The anal cancer incidence in 2012 for HIV-infected men-who-have-sex-with-men (MSM) in North America was 131 cases per 100,000 persons [8]. Anoreceptive intercourse among heterosexual women is a poorly quantified factor believed to contribute to the increase in frequency of ASCC [7].
ASCC has etiological similarities with some genital and oropharyngeal malignancies, sharing the association between cancer with HIV and HPV [9]. ASCC is associated with the oncogenic subtypes of HPV in up to 90% of the cases [2,10]. The prevalence of HPV infection in the United States ranges from between 10% to 55% in the general population and approaches 90% in HIV-infected individuals [1]. HPV infection is characterized by the over expression of viral oncogenes E6 and E7 [11]. E6/E7 oncoproteins block the tumor suppressors p53 and Rb leading to cell-cycle disruption and accumulation of chromosomal instability [11]. Increased E7 expression is also responsible for epigenetic remodeling of the p16INK4a gene locus, whose over expression results in senescence and cell-cycle arrest [11]. Anal high-grade squamous intraepithelial lesions (HSIL), caused by HPV infection, are postulated to be the precursors of ASCC [8]. HPV 16 is the most common subtype of HPV linked to anal carcinogenesis and is present in 75% of invasive ASCC [2].
Cervical cancer and ASCC are biologically similar and caused by persistent infection with high-risk types of HPV [12]. Screening strategies used in cervical cancer have been applied to anal cancer in the form of anal cytology (anal Pap smear) and high-resolution anoscopy, which is similar to colposcopy [12]. Anal cytology is performed without direct visualization of the anal canal with a moistened Dacron swab, a cervical brush or a flocked nylon swab, the implement is inserted 4cm into the anal canal and rotated around its full circumference [7]. Anal cytology is reported using the terminology and definitions of the Bethesda System [7]. The sensitivity of a single anal cytology test for detection of histological HSIL ranges from 55 to 93% and the specificity from 32 to 81% [7]. Anal cytology should be done every 12 months in HIV-positive men and women over 25- yearsold, HIV-negative MSM over 40-years-old, women with highgrade cervical or vulvar lesions or cancer over 40-years-old, men and women with perianal condyloma or HSIL over 25-years-old, and solid organ transplantation recipients or patients with other forms of immune suppression over 25-years-old [13]. High resolution anoscopy with biopsy is indicated in cases where anal cytology results are abnormal or anal cancer symptoms are present and has a sensitivity and specificity of 100% and 71% respectively [7].
Patients with ASCC can present with a variety of perianal symptoms, but usually present with a combination of pain, bleeding, swelling, pruritus, and discharge [2]. Bleeding is a common symptom for ASCC and is often attributed to hemorrhoids, delaying the diagnosis [5]. Symptoms that suggest advanced disease include fecal incontinence, pelvic pain, and the presence of a rectovaginal fistula [2]. Inguinal metastasis may be present in up to 29% of patients at presentation [2]. The diagnosis of ASCC is based on biopsy results [5]. In order to adequately stage ASCC after histological confirmation a CT scan or magnetic resonance imaging (MRI) of the chest, abdomen, and pelvis is required [2]. Endo-anal ultrasound (EUS) is useful for T stage but inadequate for N and M staging [5]. The United States National Comprehensive Cancer Network guidelines recommend PET/CT to be considered in the staging of patients with node-positive disease or T3-4 primary tumors [14].
Patients with diagnosis of HSIL can be treated with infrared coagulation or electrocautery ablation reaching cure rates for individual lesions of 67% in HIV-positive patients and 80% in HIV-negative patients [15]. HPV 16 positivity in ASCC is associated with a better outcome, being an independent prognostic factor for overall survival and disease-specific survival [3]. Historically, treatment for ASCC was abdominoperineal resection with formation of a permanent stoma [16]. Eradication of the primary tumor with preservation of the structure and function of the anal sphincters are the modern treatment goals of ASCC [2]. The actual standard therapy for ASCC is definitive chemoradiotherapy delivering 50-60 Gy with concurrent 5-FU/ MMC (17). Usual treatment for ASCC produce a 35% rate of grade 3 to 4 gastrointestinal toxicity and a 48% rate of skin toxicity [17]. Patients treated with combined chemoradiotherapy can experience ongoing distressing symptoms such as diarrhea, fecal leakage, incontinence, and poor sexual function for many years after treatment [16,18].
Capecitabine is an oral, tumor-activated fluoropyrimedine carbamate, preferentially converted to 5-FU at the tumor site, which avoids the need for hospital admission and a central line for the administration of 5-FU [19]. Patients treated with Capecitabine present less mucositis, nausea, and neutropenia than those treated with 5-FU [20]. Cost comparison of Capecitabine versus 5-FU shows that the higher drug cost of Capecitabine is offset by the costs associated with treating toxicity and administrating 5-FU (20). Intensity-modulated radiation therapy can decrease the dose administered to normal structures, while maintaining adequate ASCC coverage [18]. The 5-year survival rate for ASCC is 79%, 5-year colostomy free survival rate ranges from 65 to 82%, 5-year disease-free survival rate is 58%, and 5-year progression-free survival rate is 78% [2]. Salvage surgery can achieve local pelvic control in approximately 60% of cases with a 5-year survival rate of 30 to 60% [5]. There are no definitive guidelines on anal cancer follow-up after chemoradiotherapy [2].
Conclusion
Our patient presented to the emergency department with a combination of perianal symptoms, including the presence of a mass, fistula, pain, discharge, and bleeding, which are all suggestive of anal cancer. The diagnosis of ASCC was made by biopsy-proven histology taken during a rectal exam under anesthesia. In order to adequately stage the tumor we performed a CT scan of the abdomen and pelvis, along with a full vaginal exploration. The ASCC was classified as T3N0M0 with clinical stage II. HIV and HPV infection were ruled out because of the association of these viruses with ASCC. Our patient was treated with chemoradiotherapy using Capecitabine instead of 5-FU to avoid the need for hospital admission and a central line. Although there is no definitive guideline on anal cancer followup, we performed a PET/CT 4 months after treatment ended and will carry out clinical examination every 3 months with repeat imaging every 6 months with a low threshold for biopsy. There is no evidence of persistent or recurrent disease 7 months after treatment.
For more Open Access Journals in JuniperPublishers please click on:
https://www.linkedin.com/company/juniper-publishers
For more articles in Open Access Journal of Case Studies please
click on: https://juniperpublishers.com/jojcs/
To know more about Open Access Journals Publishers
To read more…Fulltext please click on: https://juniperpublishers.com/jojcs/JOJCS.MS.ID.555621.php



No comments:
Post a Comment