Juniper Publishers- Open Access Journal of Case Studies
Effect of Low Dose Type I Fish Collagen Peptides Combined or not with Silicon on Skin Aging Signs in Mature Women
Authored by Luc Duteil
Abstract
The effects of low dose (2.5gj type I fish collagen peptides(CP) combined (CP/Si) or not (CP) with silicon (choline-stabilised orthosilicic acid) were investigated in this double-blinded, randomized, placebo-controlled supplementation study on the skin of 57 mature women after 12 weeks of daily intake. Elasticity, hydration, thickness and color of the skin were measured objectively at baseline, Week 8 and Week 12 on face and forearm. Clinical evaluations of facial wrinkles, radiance and complexion homogeneity were performed at each visit. Significant results concerning increased elasticity and firmness were observed after 8 and 12 weeks for both CP groups and not for the Placebo group. Skin thickness was significantly increased at Week8 and Week 12 for the CP group compared to placebo. A significant increase of skin lightness (L*j was observed at Week 12 on the face for the CP/Si group. Significant clinical improvements were also observed on the face for both CPs.
Keywords: Low dose fish collagen peptides; Silicon; Nutrition; Skin elasticity; Anti-aging
Introduction
Clinical signs of dermal atrophy and skin aging (including skin dryness, hypo/hyperpigmentation, diminished skin elasticity and firmness) are associated with a reduction and disorganization of collagen [1,2]. Major changes of photoaging occur in the dermis. In photoaging, a profound decrease in collagen, glycosaminoglycans and proteoglycans is observed combined with a degeneration of elastic fibres (elastosis) resulting in a rough leathery skin surface with fine and coarse wrinkles [3]. Scanning electron microscopic examination has shown that the number and width of collagen fibre bundles decreases with age [4]. Collagen, particularly Types 1 and 3 collagen, is the major structural protein present in human skin [5,6].
Animal experiments, preclinical and clinical human trials investigating the effects of oral supplementation with collagen peptides have indicated the possibility that dietary compounds can modulate skin function [review in [7]]. For example, Matsuda et al. [8] reported that in pigs a 9-week oral ingestion of collagen hydrolysates induced increased fibroblast density and enhanced formation of collagen fibrils in the dermis in a protein-specific manner.
Silicon (Si) has been described as having an important function in the formation and maintenance of connective tissue [9]. It is involved in collagen synthesis and there is increasing evidence to suggest that it may be important for the normal health of bone and the connective tissues [10]. It has been clinically demonstrated that silicon has similar beneficial effects on skin collagen [11]. Therefore, it appears interesting to associate in a nutritional supplement a source of collagen peptides with a source of bioavailable silicon.
This present study was intended to assess the anti-aging potential of a low dose of specific fish collagen peptides (also named collagen hydrolysates) combined or not with silicon on the aging signs of the skin of mature women.
Materials and Methods
This study was conducted in accordance with the ethical principles originating from the Declaration of Helsinki and its amendments. It was carried out as a monocentric, doubleblinded, randomized, placebo-controlled supplementation study on the effects of low dose fish CP (2.5g) combined or not with silicon on the aging signs of 57 mature women after 12 weeks of daily intake. Subjects were randomly assigned to receive either type I fish CP (Naticol®, specific fish collagen peptides from Weishardt France; mean molecular weight (Mw) 2kDa) once daily or the same product combined with silicon (Naticol® / Si, mean Mw 2kDa) or the matching Placebo (maltodextrin). Measures were performed at baseline, Week8 and Week 12 on the face and on the internal and external aspects of the dominant forearm.
Skin elasticity parameters were measured using the cutometer SEM (Courage and Kazhaka, Germany). Skin hydration measurements were performed using the corneometer CM 825 (Courage & Khazaka, Germany). Skin color was measured with a Chromameter® CR 400 (Konica-Minolta, Japan) using the CIE L*a*b* color space. Skin echography evaluations were performed using a DERMASCAN C (Cortex technology, Denmark). Two skin parameters were measured: the total skin thickness and the echogenicity.
Clinical evaluation of facial wrinkles were performed by a trained clinician using the Bazin-Doublet's atlas [12]. A trained clinician used a visual analogic scale (VAS) to assess the level of skin radiance (between 0 and 10) and the homogeneity of skin complexion (between 0 and 10).
At week 12, a questionnaire was used to evaluate the volunteer's satisfaction in terms of decrease of skin wrinkles, as well as the increase of homogeneity of tone, radiance, skin hydration, firmness and brightness.
Results
The three groups were homogenously distributed according to age and skin type. Most of the subjects were skin type III (86%) and menopaused (61%). Significant results concerning biomechanics parameters were observed at week8 and week 12 for both CP groups and not for the Placebo group. Globally after 12 weeks, significant variations compared to baseline and expressing an increase of skin elasticity and firmness were the following:
a) A decrease of the residual deformation (Uf-Ua) for both CP/Si (-20% p=0.006) and CP (-17%, p=0.022) on the external part of the forearm (Figure 1).
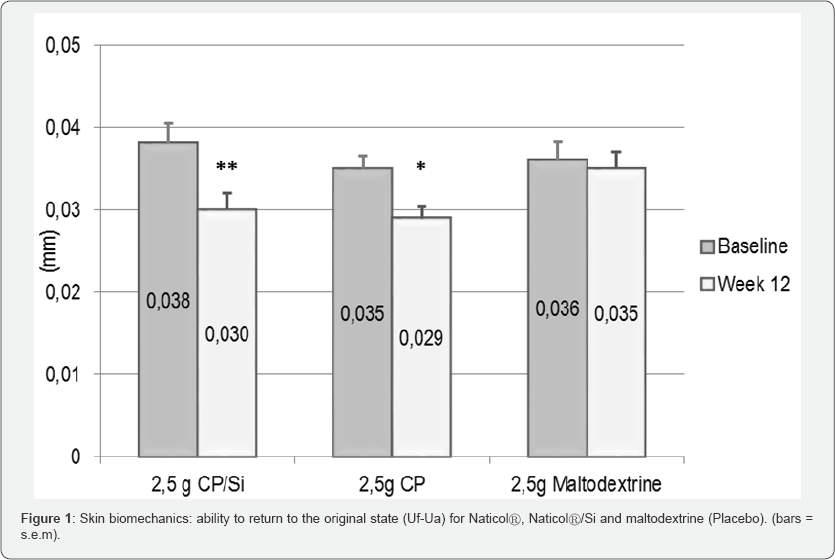
b) A significant decrease (p<0.001) of the residual deformation, the skin "fatigue" at the end of the measuring cycle (Uf-Uf5) on both the external (-21%) and internal parts (-16%) of the forearm for CP.
c) A significant increase (+10%, p=0.018) of the ratio of elastic recovery to the total deformation (Ur/Uf) for CP/Si on the internal part of the forearm.
d) A significant increase (+8%, p<0.028) of the overall elasticity at the 5th cycle (Ua5/Uf5) for CP/Si on the internal part of the forearm.
At Week 8 on the face, the variation of skin thickness vs. baseline was significantly different (p=0.019) between CP (Δ=+36μm) and placebo (Δ=-64μm) groups. At Week 12, the difference between CP (Δ= 0μm) and placebo (Δ= - 87μm) was still significant (p=0.048). There was no significant effect detected on skin hydration whatever the treatment.
Concerning the clinical assessment ofthe crow's feet wrinkles at Week 12, the score was significantly decreased by -16.5% for CP compared to baseline. At Week 12, an improvement of crow’s feet was observed in 74% and 60% of the subjects for CP and CP/Si respectively.
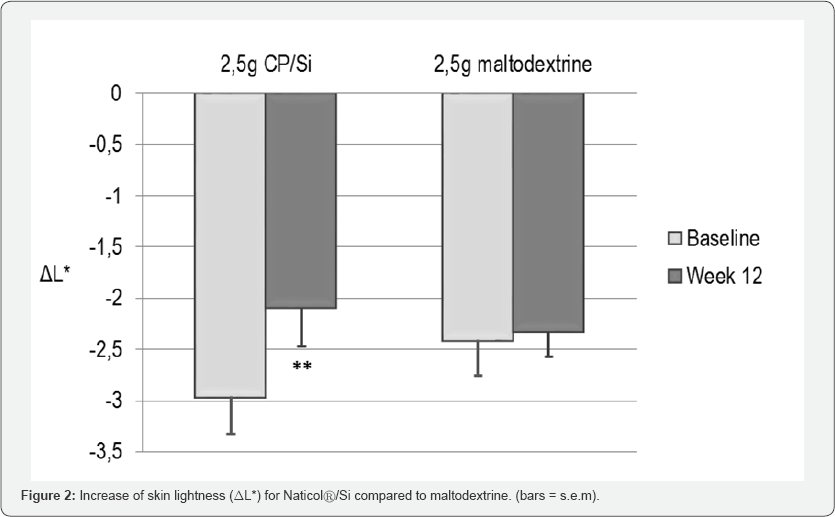
The skin tone homogeneity was significantly improved (p<0.001) at Week8 for CP compared to baseline. The skin radiance was also significantly improved for CP at Week 8 (p=0.034). These improvements were no more significant at Week 12. At Week 12, a significant increase of skin lightness (L*, p=0.004) was observed on the face of the CP/Si group (Figure 2).
Self-questionnaire: at week 12, for the CP/Si group, 79% of subjects found an increase of skin brightness and 74% found an increase of radiance on their face. For the CP group, 64% of subjects found an increase of radiance and 68% found an increase of skin hydration of their face.
Discussion
The reason of testing a low dose of CP comes, first of all from the fact that there are some expectations and demands from the consumers for the development of gummies containing nutritional supplements. On the other hand, this type of formulation doesn't allow to incorporate high doses of collagen peptides (no more than 0.5-0.8g). This limit is acceptable because the gummies are usually taken several times a day and then cumulated high dose can be avoided. Secondly, some clinical studies have demonstrated that low doses of CP could have interesting positive effects on the skin. For example, Proksch et al. [13] assessed the effectiveness of CP derived from porcine type I collagen on the skin 69 women aged 35-55 years and receiving 2.5g or 5.0g of CP or Placebo once daily for 8 weeks. At the end of the study, skin elasticity in both CP dosage groups showed a statistically significant improvement in comparison to placebo. Skin roughness measured by image analysis of skin imprints and skin hydration (corneometer®) did not detect any significant improvements.
In another double-blind, placebo-controlled study involving 114 women (45-65 years), Proksch et al. [14] showed that the ingestion of specific bioactive collagen peptides derived from porcine type I collagen (2.5g, once daily for 8 weeks) promoted a statistically significant reduction of eye wrinkle volume (p < 0.05) in comparison to the placebo group after 4 and 8 weeks (20%). Additionally, after 8 weeks of intake, a statistically significant higher content of procollagen type I (65%) and elastin (18%) was detected in CP group compared to the placebo.
Up to now, except for these two studies, no placebo controlled trials have investigated low dose CP effects (≥2.5g/ day) and in particular concerning type I fish collagen peptides associated with silicon. Concerning the effects of choline-stabilised orthosilicic acid on the skin, a randomized double-blind, placebo-controlled trial was performed [11]. Fifty women with photo damaged facial skin were administered orally 10 mg Si/day in the form of choline-stabilised orthosilicic acid pellets or a placebo for 20 weeks. Non-invasive methods were used to evaluate skin micro-relief, hydration and mechanical anisotropy. Compared to placebo, the results indicated a significant reduction of skin roughness in the choline-stabilised orthosilicic acid treated group (Rm: -19%) also on the fragility of the hair and nails (VAS assessments of brittleness) and improvement of skin isotropy and higher serum Si concentration.
The results we obtain in the present study corroborate those of a previous study we performed [15] to assess the antiaging potential of three fish collagen type I peptides (Naticol®) during 8 weeks on skin aging signs in sixty mature women aged 45-69 years. At Week 8, compared to placebo, a significant improvement of skin firmness and elasticity and a significant decrease of face wrinkle score were observed for the three CPs.
Conclusion
This present study which consisted in a once daily treatment for 3 consecutive months indicated that oral supplement containing low dose specific collagen peptides combined or not with silicon appear to have some interesting effects on skin qualities. An extension of the treatment duration to four months or more could confirm these preliminary results.
For more articles in Open Access Journal of Case Studies please click on: https://juniperpublishers.com/jojcs/index.php



No comments:
Post a Comment