Juniper Publishers- Open Access Journal of Case Studies
Male with 45,X/46,X(r)Y Mosaicism due to a Ring Y Chromosome: A Case Report
Authored by Soumya Nagaraja
Abstract
The clinical, molecular, and cytogenetic findings in a boy with 45,X/46,X(r)Y mosaicism are described here. He has history of ring Y chromosome mosaicism diagnosed by amniocentesis performed due to advanced maternal age. He was treated for short stature and growth failure with growth hormone therapy. He was transferred to our care at 12 years of age. On presentation, he had a normal male phenotype, short stature, palpable testes and delayed sexual development. A post-natal karyotype and chromosomal SNP microarray revealed deletions of both terminal regions of the Y chromosome, consistent with the prenatal diagnosis of the ring Y chromosome. On karyotype, the presumptive ring Y chromosome was present in 29% of the cells and a single X chromosome was present in the other 71% of cells. FISH analysis demonstrated the presence of a ring Y chromosome in 37.1% of the cells. SHOX gene analysis revealed a complete gene deletion and is the likely cause of his short stature. He continued treatment with growth hormone and an aromatase inhibitor was added to delay growth plate fusion and to potentially increase his final adult height. The 45,X/46,X(r)Y mosaicism has been rarely reported. This mosaicism, due to the presence of the ring Y chromosome and depending upon on the presence or absence of the SRY gene can result in a wide spectrum of manifestations ranging from females with a Turner syndrome-like phenotype to phenotypic males. This karyotype can be associated with mixed gonadal dysgenesis in both boys and girls, depending on the tissue distribution of the 45,X and 46,X(r)Y cell lines. As the phenotype in mosaicism depends on the proportion of each cell line, analysis of other tissue types (other than lymphocytes) is needed to test the level of mosaicism and study its effect on the sexual differentiation and phenotype.z
Keywords: 45,X/46,X(r)Y mosaicism; Ring Y chromosome
Abbreviations: FISH: Fluorescent in Situ Hybridization; GH: Growth Hormone; ISS: Idiopathic Short Stature; SRY gene: Sex Determining Region Y; SHOX gene: Short Stature Homeobox Gene
Introduction
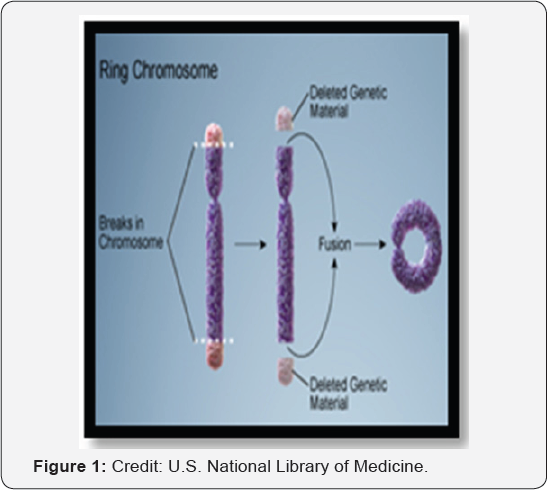
A ring chromosome is an extremely rare chromosomal aberration, which can occur in autosomes and sex chromosomes. The frequency of a ring chromosome in clinically detectable conceptions is 1/25,000 however, the incidence of the ring Y chromosome is unknown [1]. The formation of a ring Y chromosome involves terminal breakage in both chromosomal arms (p and q arms) and fusion of the resulting ends with loss of the deleted distal material (Figure 1). Because of the instability of a ring Y chromosome during mitosis, a second monosomic cell line is often present in these patients [2]. There are several mosaicisms reported with ring Y chromosomes including 45,X/46,XX/46X(r)Y, 45,X/46,XY/46,X(r)Y, 46,XX/47,XX(r) Y, 45,X/46,X(r)Y, 45,X/46,X(r)Y/46, X, dicr Y [3]. Patients with mosaic 45, X/46,X(r)Y karyotype, present with phenotypes ranging from females with Turner-like phenotypes, phenotypic males and females with mixed gonadal dysgenesis, to almost phenotypic normal males [4,5]. Variability of phenotype depends on the percentage of monosomic cells in different tissues and on the genetic material deleted during the formation of ring Y chromosome. There are very few published reports on 45,X/46, X(r)Y mosaicism. In most of the reported cases, patients have presented later in life due to infertility. Few of the reported cases in children have presented with clitoromegaly, chordee, cryptorchidism, hypospadias, and short stature. Here, we present the molecular cytogenetics of 45,X/46,X(r)Y mosaicism in a 12-year-old boy with normal male phenotype, chordee, short stature andpubertal delay.
Clinical Case Report
A 12-year-old boy of healthy and non-consanguinous parents was transferred to our care for management of short stature. He was being treated with growth hormone (GH) therapy for short stature and growth failure since 8 years of age. He was born at term after a normal pregnancy and delivery. He had history of ring Y chromosome mosaicism diagnosed by amniocentesis performed due to advanced maternal age. He had chorde eat birth and underwent surgical correction. His motor and mental development was normal. On examination his height was 139.5cm (8th centile). His weight was 37.4kg (32%), body mass index (BMI) was 19.2kg/m2 (70th centile). He was a well- nourished boy without any dysmorphic features. His external genitalia appeared predominantly male, his penis was 4.5cm, and right testis measured 2cc and the left testes 1cc with no axillary or pubic hair.
Initial amniocentesis showed 45,X karyotype in all cells. Prenatal ultrasound revealed a male phenotype and thus the specimen was re-evaluated by fluorescence in situ hybridization (FISH) analysis showing the presence of a ring Y chromosome. Since his prenatal chromosomal analysis was conflicting, we performed post-natal chromosomal analysis which revealed mosaicism 45,X/46,X(r)Y. On analysis, 71% of the cells showed a single X chromosome and 29% of the cells showed the presumptive ring Y chromosome. FISH for the Y chromosome showed that it was present in 37.1% of cells. Microarray showed deletion at both ends of the Y chromosome with a presumptive ring Y chromosome.
Analysis ofthe SHOX gene showed that there was a whole gene deletion. The SRY gene was present on the ring Y chromosome that contributed to his male phenotype. Ultrasound of the abdomen showed a horseshoe kidney but there was no gonadal dysgenesis. The echocardiogram and the EKG were normal. There were no other stigmata of Turner syndrome. Thyroid analysis showed TSH 9.47uIU/ml with normal thyroid hormone levels. Both thyroid peroxidase and antithyroglobulin antibodies were positive. Levothyroxine was added to GH therapy. He showed a good response to treatment and improvement in his height however, he continued to have delayed pubertal development. Since there was advancement of his bone age, anastrazole was added to delay growth plate fusion and to potentially increase his final adult height. Eventually, there was improvement in his gonadarche. At 14 years of age, his testicular size increased to 6cc bilaterally and complete biochemical analysis along with hormonal assessment was performed and was suggestive of hypergonadotrophic hypogonadism. At 16 years of age, semen analysis was done to test spermarche and he was noted to be azoospermic. These findings were consistent with deletion of the AZFa gene during the ring Y-chromosome formation. The AZFa gene is essential for spermatogenesis. The fertility potential in this patient cannot be determined at this point. A GnRH stimulation test and repeat semen analysis will be performed after discontinuing anastrazole and GH therapy
Prenatal chromosomal analysis and FISH studies on cells in the Amniotic fluid
In the initial cytogenetic evaluation of the amniotic fluid, an abnormal female karyotype was found in which all the cells examined contained a single X chromosome consistent with the diagnosis of Turner syndrome. Subsequent ultrasound of the fetus suggested a male phenotype. Therefore, FISH studies were performed on the interphase cells preserved from amniocentesis using the Vysis, sex chromosome probes: CEP1 which is specific for the X chromosome centromere, SRY which is specific for Yp11.3, and CEPY which is specific Yq12. These FISH studies showed a very low-level mosaicism for the hybridization to the Y chromosome probes. Two of the 100 cells showed male pattern of hybridization with the SRY probe. Two of the additional 100cells showed an unusual but positive hybridization to the Y centromere and long arm probes. Three cells contained small ring-shaped chromosomes that likely represented a ring Y chromosome. The SHOX gene was completely deleted on the Y chromosome but the SRY gene was present. The AZFa locus was deleted but the AZFb and AZFc loci were preserved. Its effect on the phenotype remains unexplained.
Post-natal chromosomal analysis
Post-natal chromosomal analysis showed 45,X/46,X(r)Y chromosome mosaicism. Both karyotype are shown below. The ring Y chromosome is indicated by the arrow (Figure 2 & 3).


FISH and microarray studies
Whole genome SNP microarray revealed both a terminal Yp and Yq deletion. There was a 1.02MB terminal deletion of the YPTER- P11.32 and 34.6MB terminal deletion of YQ11.223- QTER. This was consistent with ring Y chromosome. The presumptive ring Y chromosome was present in 29% of the cells and only a single X chromosome was present in 71% of the cells. Concurrent FISH analysis demonstrated the presence of a ring Y chromosome in 37.1% of the cells. Although the SHOX gene was deleted, the SRY gene was present on the ring Y chromosome that may have contributed to his male phenotype.
Semen analysis
At age 16, semen analyses were performed. Uncentrifuged and centrifuged semen specimens were examined microscopically and zero sperm cells were seen.
Discussion
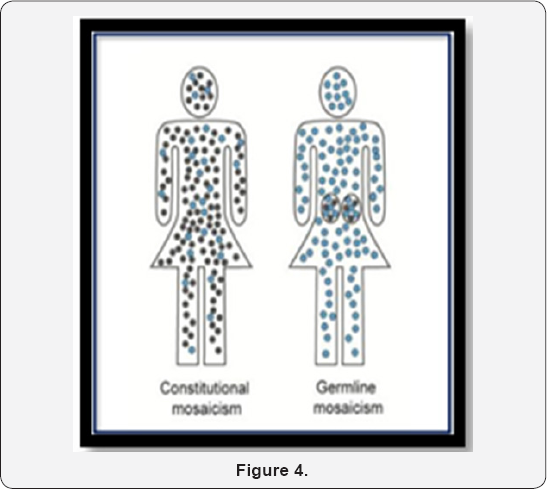
Mosaicism is the presence of two or more populations of cells with different genotypes in one individual who has developed from a single fertilized egg [6]. Mosaicism can be seen in the somatic cells or the germline cells. In somatic mosaicism (Figure 4), different somatic cells of the body have more than one genotype. Different genotypes arise from same single fertilized egg cell due to mitotic errors at initial or later cleavages. In some rare cases, disorders of sexual development can be caused by mosaicism where some cells in the body have XX and other have XY chromosomes.
In germline mosaicism (Figure 4), the diploid germ cell precursors in the gonad (either sperm or oocytes) are heterogeneous. Some may have a mutation and some may be completely normal. A mosaic germline mutation is very important because it can be passed to offspring. Germline mosaicism can be observed with any inheritance pattern but it is most commonly seen with autosomal dominant and X linked disorders. Most individuals are unaware they possess germline mutations until they have an affected offspring [7].
Genetic mosaicism can be confused with chimerism in which two or more genotypes arise in one individual similarly to mosaicism. However, the two genotypes arise from the fusion of more than one fertilized zygote in the early stages of embryonic development, rather than from a mutation.
Here we present a male patient with a mosaic karyotype with a high percentage of monosomic 45,X cell line and small ring (Y) variants in his karyotype. The high percentage of the monosomic 45,X cell line in our patient, is seen due to the instability of the ring chromosome during mitosis. Molecular analysis showed deletion of the SHOX gene with the presence of the SRY gene on the ring Y chromosome, which explains his short stature and male phenotype, respectively.
Bettio et al. [8] indicated that phenotypic sex is determined by the presence of the 45,X vs. SRY-bearing cells and depends more on the number of copies of the SRY gene rather than on the percentage of 45,X cells, in the gonads. Thus in our patient, although the majority of cells have a 45,X karyotype, the phenotype is male and this could be explained by an enrichment of the 46,X(r)Y karyotype in the gonads.
The phenotypic spectrum of males with a 45,X/46,X(r)Y karyotype and bilaterally descended testes varies greatly from males with short stature and spermatogenic failure to males with normal stature and less severely affected spermatogenesis [9,10]. There has been a report of a 45,X/46,X(r)Y male with normal male phenotype, oligospermia, who fathered a son via in vitro fertilization [11]. Some of the case reports have reported subtle signs of Turner syndrome with male phenotype. Even with absence of Turner stigmata, cardiac and renal anomalies must be excluded in all such cases. It is also important to check for gonadal dysgenesis in these patients as it is associated with increased risk of gonadoblastoma.
The findings in this patient with 45,X/46,X(r)Y raise important questions about differing genetic mechanisms leading to the varied clinical spectrum of presentation. Previous findings from the prenatal amniocentesis prompted us to recheck the post-natal karyotype, SHOX gene and SRY gene in this patient, which would have been otherwise missed considering there were no associated characteristic features of a disease or syndrome in this patient except short stature, chordee and delayed puberty.
In pediatric endocrinology practice, deletions of the entire SHOX gene or mutations within or near this gene have been identified in some patients with short stature. However, there are many other genetic causes of short stature which go undiagnosed. These patients with short stature are grouped under the diagnosis of idiopathic short stature (ISS) and have no characteristic features of a known disease or syndrome. Cases like ours prompt us to think about the need for a thorough genetic evaluation to uncover the underlying cause of ISS so that appropriate treatment is provided. Performing karyotype, FISH and microarray can help to delineate the underlying genetic defect (including mosaicism and other chromosomal aberrations), which can affect not only growth and puberty, but also sexual development and maturation.
More case studies will give us a better understanding of how to treat children with short stature and hypogonadism as a result of mosaicism due to ring Y chromosome.
Conclusion
As the phenotypic presentation in mosaicism depends on the proportion of each cell line, analysis of other tissue types (other than lymphocytes) needs to be studied to test the level of mosaicism and to understand its effect on the sexual differentiation, phenotype, growth and development.
For more articles in Open Access Journal of Case Studies please click on: https://juniperpublishers.com/jojcs/index.php



No comments:
Post a Comment