Juniper Publishers- Open Access Journal of Case Studies
Peripartum Cardiomyopathy Acute Heart Failure: The First Case Report of C Protein as Adjuvant Anticoagulation Therapy in Urgent Heart Transplantation
Authored by Marzia Cottini
Abstract
We described the case of a woman affected by peripartum cardiomyopathy, complicated by acute heart failure, cardiac intracavitary and venous thrombosis. She had a coagulative disorder (Protein C deficiency), and she was unresponsive to medical therapy and mechanical circulatory support. Because of that, she was successfully transplanted after C protein adjuvant anticoagulation therapy. This is the first reported case of the C protein replacement in order to perform urgent heart transplantation in peripartum life-threatening cardiomyopathy with coagulative disorders.
Keywords:
Keywords: Antioxidant; Apoptosis; Cerebral cortex; Neurotoxin; Nutmeg
Introduction
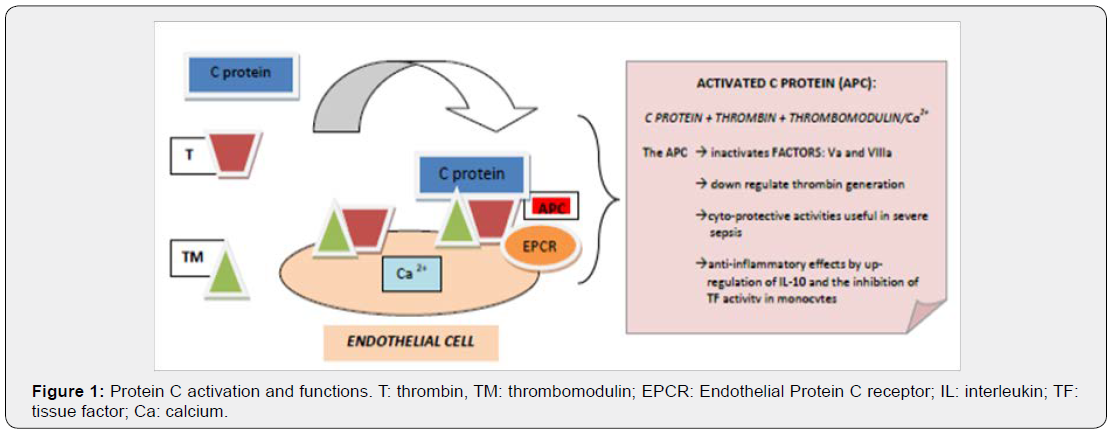
Peripartum cardiomyopathy (PPCM) was characterized by severe systolic dysfunction which can affect women in last month of pregnancy until up five months post-delivery, in absence of other identifiable causes[1,2].Survival rate of severe PPCM were very low in particularly if evolved to acute heart failure requiring advanced medical treatment, mechanical circulatory support (Extracorporeal Membrane Oxygenation [ECMO] or Left Ventricular Assist Device [LVAD], and so on) and rarely cardiac transplantation[3,4]. Moreover, pregnancy was associated with coagulative disorders: increasing of fibrinogen and Factor VIII levels, acquired deficiencies of coagulative C protein and Antithrombin (Figure 1). Finally, the combination of severe ventricular dysfunction and coagulative disorders could promote the development of thrombi. Informed and written consent was obtained from the patient.
Description of Case
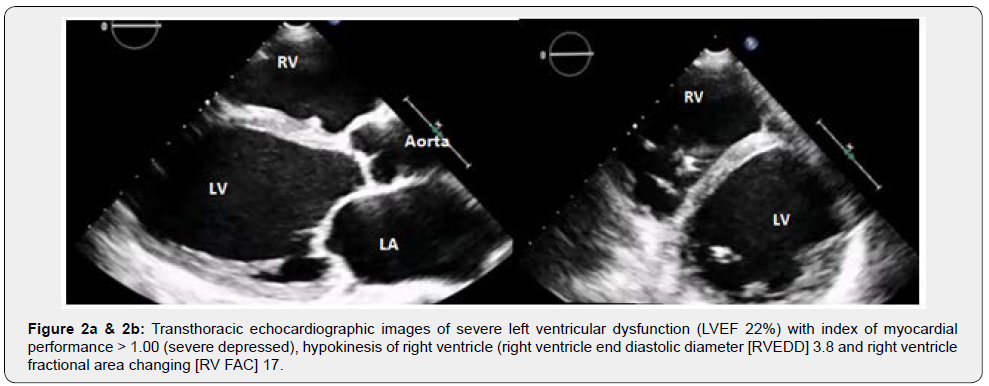
A 27year-old female (Body Surface Area [BSA] 1.67m2) developed progressive dyspnoea (NYHA functional class IIIb) 55 days after giving birth (spontaneous full-term delivery). She was admitted to Intensive Cardiology Unit and underwent to TransThoracic Echocardiogram (TTE) then Cardiac Magnetic Resonance Imaging (MRI) that documented severe left ventricular dysfunction (Left Ventricle Ejection Fraction [LVEF] < 25%, Figure 2a), hypokinesis of right ventricle (RV) (Figure 2b, Video 1). As a prophylaxis of sudden arrhythmic death, the patient received an implantable cardioverter defibrillator (ICD). The patient was treated with diuretics, vasodilators and oral anticoagulant therapy overlapping low molecular weight heparin (LMWH) until the achievement of therapeutic INR.After one month, she presented hepatic dysfunction secondary to heart failure, hence she was transferred to our Cardiovascular Intensive Care Unit with unstable hemodynamic (Arterial Pressure 80/50mmHg, Heart Rate 115 beats/minute), lactate acidosis (Lactate 4.5mmol/l) and slight dyspnoea. We started infusion of dobutamine (5mcg/kg/min), nitroprussiate (0.1mcg/kg/min) and furosemide (20mg/hour). Lab data evidenced severe deficit of antithrombin (AT III 26%) and coagulative C protein (36%), high levels of Factor VIII (>200%), DDimer test (3.618ng/ml), and Fibrinogen (390mg/dl) (Table 1). Thrombophilic screening showed eterozygotes phenotype for methylenetetrahydrofolate reductase (MTHFR) with normal homocysteinemia (6.3micron/l). The patient’s group blood was A, the test for Heparin Induced Thrombocytopenia (HIT) was initially negative.

LVEDV: Left Ventricular End Diastolic Volume; BSA:Body Surface Area; LVEF: Left Ventricular Ejection Fraction; C.I.: Cardiac Output Indexed for BSA (echocardiographic measure); AT: Antithrombin; CCP: Coagulative C Protein; WBC: White Blood Cells; C-RP: C Reactive Protein; BNP: Brain Natriuretic Peptide; Trop I: Troponin I
An ultrasound Echography detected multiple thrombosis of both jugular internal veins, of right axillary and subclavian veins. A control TTE confirmed dilated evolution of PPCM with further thrombus around the right wire of ICD. We required a consultation with a haematologist and decided to replace Antithrombin and C protein deficiency with protein concentrate (Ceprotin®, Baxter Bio Science Glendale USA) 50UI/kg every 24 hours, after initial bolus of 100UI/kg, in combination with fondaparinux 2.5mg twice. It was also administered Levosimendan (0.1mcg∙kg -1.min- 1) and metylprednisolone 1gr/day for three days, followed by 1mg/kg/day and progressive de-escalation.
After two weeks, her clinical condition rapidly evolved to cardiogenic shock associated with increase of lactate acidosis (7.7mmol/l). Adrenalin was associated with dobutamine infusion and intraortic balloon pumping (IABP) was positioned. The patient was intubated because of arrhythmic storm (ventricular fibrillation) and worsening of hemodynamic parameters. Considering the deterioration of hemodynamic and respiratory values, the unresponsiveness to maximal medical therapy, the impossibility of ECMO or LVAD implantation because of the aggravation of coagulative disorders, the heart team decided for the last therapeutic choice: heart transplantation. Considering the thrombocytopenia (36.000/mm3), the test of HIT was repeated and a phosphorylcholine coated circuit and membrane oxygenator (Sorin Group Deuschland GmbH, Munich, Germany) were used for cardiopulmonary bypass (CPB). The monitoring of anticoagulation was supported by thromboelastometry and measuring Activated Clotting Time (ACT) both. The anticoagulation was performed with intravenous administration of Heparin 25000UI (400U/kg), Antithrombin 1000UI and with Concentrate C protein 2000UI (30UI/kg), to achieve ACT target of 500sec. The surgical procedure was free from complications. The haemostasis was characterized by protamine hydrocloride and platelet concentrates administration with the guidance of thromboelastometry. Adrenalin infusion was rapidly discontinued, the estubation was performed 24 hours after surgery. IABP was removed after three days. The patient was discharged from ICU with oral anticoagulant therapy (warfarin) ten day after transplantation, with AT, protein C and PLTs values normalized and D Dimer value progressively decreased.
Discussion
The pregnancy was a period of hypercoagulability conditions: it was normally characterized by high levels of fibrinogen and factor VIII that increasing the risk of venous and arterial thrombosis during pregnancy and puerperium both[5,6].The plasma factor VIII circulated as a complex with von Willebrand Factor (vWF) whose level depended on endothelial stimulation and blood group. There was a narrow relationship between blood groups A, AB and higher plasmatic levels of factor VIII, vWF and thrombotic events than blood group O[5].
Hypercoagulability state could also occur for deficiency of C protein due to the lack of Factor V inactivation (Figure 2) [7].
Our patient showed the coexistence of those factors: blood group A, high plasmatic value of Factor VIII, C protein and AT deficiency. Those deficits could be due to combination of pregnancy/puerperium hormonal state according to scientific literature, experience and haematologists’ opinions[8-12].
The management of anticoagulation for CPB was carefully planned because of the high index of suspicion for HIT and was performed with the combination of Heparin, AT and C protein bolus. We didn’t administer tranexamic acid because of preexisting thrombosis and no detection of hyperfibrinolysiswith thromboelastometric assay.
Not everyoneconsider a protein C level of 36% sufficiently low to necessitate replacement but in this special case, the clinical and lab data suggested our team and specialist to choice this specific therapeutic option: replace C protein and AT in combination with anticoagulant therapy.
The role of C protein replacement was especially important in this case:
a) firstly, to avoid venous thrombosis due to the absence of protection of activated C protein in the exposure of procoagulant proteins and platelet phospholipids to the vessel wall in the slow-flowing venous circulation;
b) secondarily, to increase the potential role of antiinflammatory and cytoprotective functions of its link with protease-activated receptor-1 (PAR-1) during the CPB;
c) thirdly, to regulate the intrinsic and extrinsic pathway of the coagulation by the control of the activation of factors V and VIII.
For more articles in Open Access Journal of Case Studies please click on: https://juniperpublishers.com/jojcs/index.php



No comments:
Post a Comment