Juniper Publishers- Open Access Journal of Case Studies
Comparison of Periodontal and Peri-implant Microflora in Smokers
Authored by Abubekir Eltas
Abstract
Aim: In this study, it was aimed to compare microbiological findings obtained from implants and teeth of individuals with dental implants in function for at least 3 years with respect to presence of smoking.
Material and Methods: According to the study protocol, individuals included in the study were divided into 2 groups; non-smoking healthy individuals (group 1) and smoking helthy individuals (group 2). Probing depth (PD) measurements were performed for all implants and teeth of the study subjects. Subgingival microbiological samples were taken from the implants and teeth for the evaluation of the content of subgingival microflora. The samples obtained were analyzed by PCR (polymerase chain reaction) method. For comparing clinical and laboratory findings, Oneway Anova test was used for quantitative data whereas Chi-square test, Fisher’s Exact Chi-square test, Continuity (Yates) correction and Fisher Freeman Halton test were used for qualitative data.
Results: The incidence of Porphyromonas gingivalis, Prevotella intermedia, Tannerella forsythia among the microorganisms examined was similar in both groups (p> 0.05). Treponema denticola species were found to be statistically significantly higher in smoking group. (p <0.05>). In the both groups, the distribution of the microorganisms was similar both on teeth and implants (p> 0.05).
Conclusion: In conclusion, our findings suggest that periimplant and periodontal microflora composition was similar at long-term implants in smoking and non-smoking individuals.
Keywords: Dental implants; Microbiology; Cigarette
Introduction
Implant treatment is an effective and valid treatment option to replace missing teeth in total or partial toothless patients. Many studies have shown high success rates in the long-term outcome of implant treatment in the general population. The preservation of the health of the peri-implant tissues also plays an important role in implant success [1].
Pathological changes in peri-implant tissues are called peri-implant diseases. The etiology of peri-implant diseases and the etiology of periodontal diseases are similar. Weak oral hygiene, Diabetes Mellitus, smoking and history of periodontitis are known as risk factors for peri-implant diseases [2].
Smoking increases the prevalence of peri-implantitis like periodontitis. First, Bain and Moy investigated the relationship between smoking and implant failure. They compared between smokers and nonsmokers and found the rate of failure of 5.92% in non-smokers and the rate failure of 11.28% in smokers [3]. It is thought that this increase in the rate of implant failure in smokers may also be affected by a more pathogenic oral flora. Therefore, peri-implant sulcus microflora is an important factor affecting the success of the implant [4].
For many years, researchers have investigated the causes of periodontal disease and have conducted microbiological studies to identify microorganisms that cause disruptive periodontal disease [5]. It was emphasized that these microorganism complexes can initiate and sustain disease in an easily affected host [6-8].
The pathogens observed in the microbial flora in the peri-implant lesions were identified by Socransky and were similar to those in the periodontitis which are red complex species (Porpyhromonas gingivalis, Treponema denticola, Tannerella forsythia) and orange complex species (Fusobacterium and Prevotella intermedia) [9]. Agregatibacter actinomycetemcomitans (A.a) have also been reported to be isolated in peri-implantitis lesions [10,11].
The reduction of local oxygen pressure in smokers results in colonization and growth of anaerobic bacteria. Therefore, it has been reported that the most suitable environment for the growth of anaerobic pathogens has become deep periodontal pockets where oxygen decreases [12].
The aim of this study was to evaluate the periodontal and periimplant sulcus microflora in smokers and non-smokers.
Material and Method
This cross-sectional study was carried out in smokers and nonsmokers healthy individuals receiving dental implant treatment at the Department of Periodontology of Faculty of Dentistry, Inonu University between 2010 and 2015. Participants selected in accordance with the study criteria were educated about the purpose and method of study, and then a written consent form was obtained from each participant prior to the initiation of research protocols.
Patient selection
Volunteers meeting the following inclusion criteria were included in the study: having at least one dental implant, having an implant functioning for at least 3 years, no systemic disease that may affect periodontal parameters.
The exclusion criteria were history of chronic systemic disease, oral cancer or non-healing lesion, osteoporosis-osteopenia or any bone malformation, active oral infection, use of antibiotics and steroids within the last 3 months, history of periodontal treatment within the last 6 months.
Research protocol
Participants were divided into 2 groups according to cigarette smoking status: The healthy non-smokers group (group 1) and healthy smokers group (group 2). The random distribution of patients was performed by another researcher (A.E.).
Probing depth (PD) measurements of all implants and teeth were performed. Using Williams periodontal probes (Hu-Friedy, Chicago, IL, USA), measurements were performed on all implants and teeth. Microbiological analyses were then performed on subgingival microbial specimens obtained from the implants and teeth of the participants.
Collection of microbiological specimens
After clinical measurements, subgingival specimens were taken from the deepest and similar depth point of each tooth and implant pocket. The bacterial specimens were collected from the peri-implant pocket through the use of sterile paper points (Paper Points, Dia Dent, Korea). The region was isolated with sterile cotton bumpers and briefly dried with air spray. Standard sterile paper points were placed into the peri-implant and periodontal pocket entrance to the extent a slight resistance was felt. Two sterile paper points no. 40 were placed in each pocket and they remained in place for 20 seconds. While paper points were being removed from the area where specimen was collected, contact with the surrounding tissues was avoided in an attempt to prevent contamination of the specimens with saliva. Paper points were then transferred into tubes containing 2 ml of distilled water in aseptic conditions and stored at -20°C until nucleic acid isolation. removed from the area where specimen was collected, contact with the surrounding tissues was avoided in an attempt to prevent contamination of the specimens with saliva. Paper points were then transferred into tubes containing 2 ml of distilled water in aseptic conditions and stored at -20°C until nucleic acid isolation.
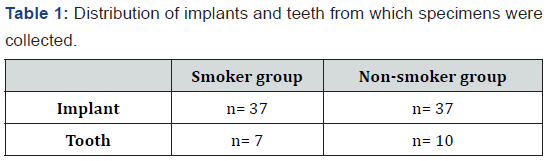
The distribution of implants and teeth from which microbial specimens were collected in each group is presented in Table 1.
Microbiological analysis
Pathogens were detected by Polymerase Chain Reaction (PZR) method. Total DNA isolation from the sample was performed using a QIA symphony DNA isolation device (QIAGEN, Germany). For DNA amplification, Qiagen TopTaq Master Mix Kit (QIAGEN, Germany) was utilized. Specific primers were employed for the identification of Porphyromonas gingivialis, Tannerella forsythia, Actinobacillus actinomycetemcomitans, Treponema denticola, Prevotella intermedia. The PZR process was performed in a volume of 25μl. Mixture contents were prepared as 12.5μl 2X master mix, 2.5μl 10X coral load, 1μl forward primer, 1μl reverse primer, 6μl H2O and 2μl total DNA.
Statistical analysis
The statistical analyses of the research data were performed on the software package IBM SPSS Statistics 22 (SPSS IBM, Turkey). One-way ANOVA test was used to compare quantitative data while Tukey’s HSD test was used to determine significant differences between groups. Chi-square test, Fisher’s Exact Chisquare test, Continuity (Yates) Correction and Fisher Freeman Halton test were used for comparison of qualitative data. A p value less than 0.05 (p<0.05>) was considered statistically significant.
Results
Microbiological findings
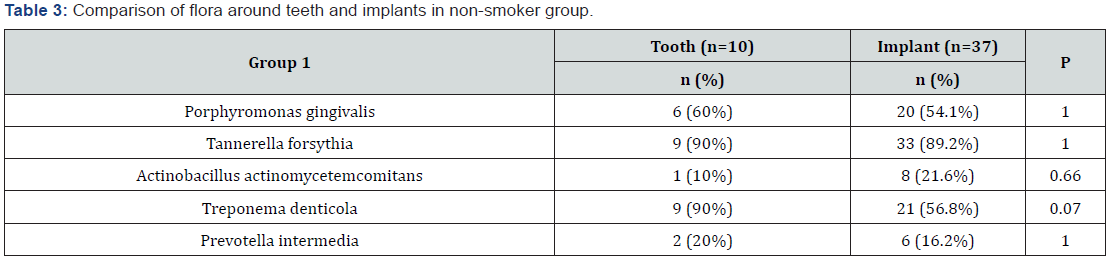
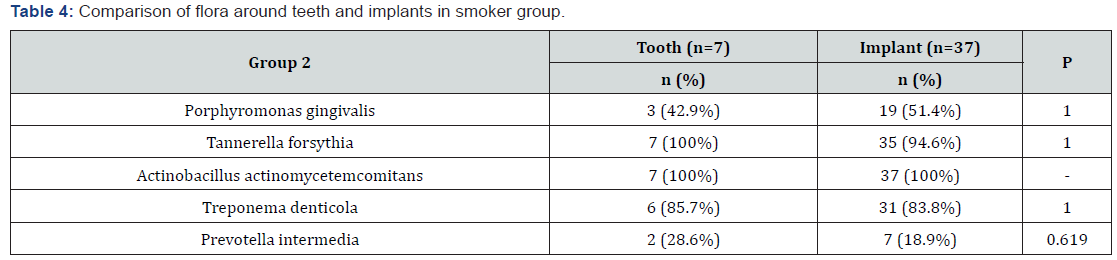
Microbiological analysis revealed that both groups were similar in terms of presence of Porphyromonas gingivalis, Prevotella intermedia, Tannerella forsythia (p>0.05). The rates of Treponema denticola species were significantly lower in non- smoker group (p<0.05>). The rates of Actinobacillus actinomycetemcomitans species were significantly lower in smoker group (Table 2). The distribution of microorganisms between teeth and implants was similar in both groups (p>0.05) (Table 3 & 4).

Discussion
In this study, the pathogen distributions in peri-implant and periodontal grooves of smoker and nonsmoker patients with systemically healthy, long-term function implants were compared.
Regular clinical control of dental implants is of great importance in the early detection of peri-implant diseases. Cigarette smoking is known to be a risk factor for periodontal and peri-implant disease. According to the literature, cigarette users have a greater pocket depth, loss of bone and attachment, and increased prevalence of periodontal disease [12,13]. Similarly, the negative effects of smoking on implant treatment have been demonstrated.
The most important factor in periodontal and peri-implant diseases is pathogenic microorganisms in microbial dental plaques. Therefore, the use of microbiological parameters has an important role in both microbial development and routine diagnosis and follow-up [14].
Pathogens such as A.actinomycetecomitans, T.forsythia and P.gingivalis are responsible for the development of both periodontitis and peri-implantitis and disease progression. In addition, many pathogens such as P.intermedia, C. rectus, P.micros, F. nucleatum and E.nodatum are involved in these diseases. In our study, microbiological analyzes were performed for the species that are often found in periodontal and peri-implant lesions, which are A. Actinomycetemcomitans, P. gingivalis, P.intermedia, T. forsythensis, T. denticola.
Compared with smokers and nonsmokers, it has been reported that there is more bacterial load in smokers. In some studies investigating the subgingival microflora of smokers, T.forsythia and P.gingivalis have been shown to be dominant [12,15,16]. Ata Ali et al. [17] evaluated T. forsythia, T.denticola, P.gingivalis and total bacterial load in dental implanted heavy smokers. It was found that smokers had deeper pockets and more complex peri-implant microbiates [17]. According to Nagaraja et al. [18], in subgingival environment of smokers, Veillonella and Streptococcus species are decreasing, while Parvinomonas, Fusobacterium, Bacteroides, Porphyromonas, Campylobacter, Treponema are increasing [18]. In addition, it is expected that the species T.denticola and T. forsythia, which are members of the red complex, will be present in the subgingival flora of smokers as it creates a suitable environment for non-Gr (-) anaerope species. In accordance with this information, in our study, T.denticola were found higher than smokers group. The incidence rates of Porphyromonas gingivalis, Prevotella intermedia, and T.forsythia species in peri-implant flora were similar in two groups.
The most important etiological factor that threatens the health of peri-implant tissues is microbial dental plaque and its content as in periodontal tissues. In this respect, the amount and pathogenicity of microorganisms in microbial dental plaque accumulated around natural teeth and implants is important [19]. Sigrun eick et al. [20] compared the microbiates between the implant and the tooth adjacent to the implant. In the study, the species which were dominant in the implants were reported to be T.forsythia, P.micra, F. Nucleatum and C.rectus, and these species were found to be dominant in smokers compared to nonsmokers [20]. Hultin et al. [10] compared the microflora of periodontal and peri-implant pockets and found similar pathogens of Prevotella nigrescens, P.intermedia, T. forsythensis, C.rectus, A. Actinomycetemcomitans in their study [10]. In our study, samples from the deepest and similar depth pockets of implants and teeth were compared, and the incidence of microorganisms in teeth and implants in smoker and non-smoker groups was also statistically similar.
Conclusion
In conclusion, our findings suggest that peri-implant and periodontal microflora composition was similar at long-term implants in smoking and non-smoking individuals.



No comments:
Post a Comment