Juniper Publishers- Open Access Journal of Case Studies
Ertugliflozin (Steglatro): New Drug in the Family
Authored by Natasha N Colvin
Abstract
Ertugliflozin, a sodium glucose co-transporter inhibitor, was approved by the FDA on December 22, 2017 for patients with type 2 diabetes. For patients requiring multiple medications to help manage their diabetes and lower their A1C, ertugliflozin may be an appropriate option. This article discusses appropriate dosing, use, and monitoring.
Keywords: Diabetes; Glucose control; Management; Ertugliflozin; Steglatro; Sodium glucose co-transporter inhibitor
Abbreviations: T2DM: Type 2 Diabetes; HbA1c: Glycosylated Hemoglobin; SGLT2: Sodium Glucose Co-Transporter 2, FDA: Food and Drug Administration; (eGFR): estimated Glomerular Filtration Rate
Introduction
Ranked as a leading cause of death in the United States, diabetes affects approximately 30 million Americans of which 90-95% has type 2 diabetes (T2DM) and one-third of those individuals are not at their glycosylated hemoglobin (HbA1c) goal [1]. The American Diabetes Association (ADA) annually publishes the global standards of medical care in diabetes to reflect upon the latest advances and improve care for people living with diabetes [2]. With many new drugs coming to market, it is important that prescribers choose the appropriate therapy to suit the patient’s needs while also helping them reaches their glycemic goals.
SGLT-2 Inhibitors
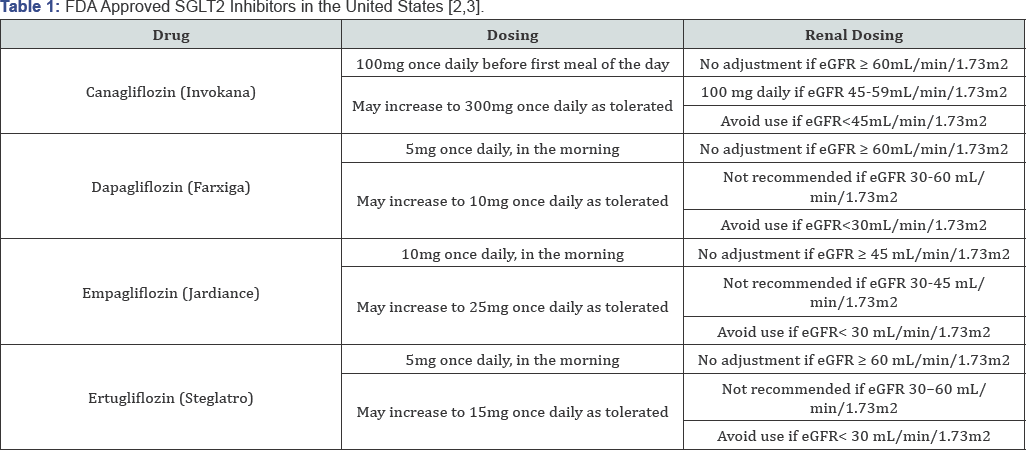
Sodium-glucose co-transporter 2 (SGLT2) is a human protein that facilitates glucose reabsorption in the proximal tubule in the kidneys [2]. A relatively new class of agents, SGLT2 inhibitors work by blocking glucose reabsorption in the proximal renal tubule by inhibiting SGLT2 [2]. Similar to most diabetes-treating medications, these agents are taken orally once daily [3]. This class of agents have the potential to provide modest weight loss and blood pressure reduction in patients with type 2 diabetes (T2DM) [2]. SGLT2 inhibitors may have glycemic benefits in patients with T2DM on insulin therapy and reduce the amount of insulin needed [2] Table 1 displays SGLT2 inhibitors that are currently Food and Drug Administration (FDA) approved in the United States.
Ertugliflozin
Ertugliflozin was approved by the FDA on December 22, 2017 as an adjunct to diet and exercise to improve glycemic control in adults with T2DM [4]. It is available in 5mg and 15mg tablets which should be used according to the dosing schedule in Table 1 [4]. Ertugliflozin is currently available at a wholesale acquisition cost of $8.94 per day which does not include discounts [4]. In the VERTIS SITA2 trial, the 5mg and 15mg dose of ertugliflozin resulted in significant HbA1c, fasting plasma glucose, body weight and systolic blood pressure reductions when used in combination with metformin plus sitagliptin [4]. HbA1c reductions were 0.7% with the 5mg dose and 0.8% with the 15mg dose compared to 0.2% with placebo [4]. Fasting plasma glucose reductions were 25.7mg/dL with the 5mg dose and 32.1mg/dL with the 15mg dose compared to 6.5mg/dL with placebo [4]. Body weight reductions were 6.6 pounds with the 5mg dose and 6.2 pounds with the 15mg dose compared to 2.2 pounds with placebo [4]. Systolic blood pressure reductions were 3.8 mmHg with the 5mg dose and 4.5 mmHg with the 15mg dose compared to 0.2mmHg with placebo [4].
Adverse Reactions
The most common side effects associated with ertugliflozin are female genital mycotic infections which had an observed incidence of ≥ 5% [5]. Other warnings and precautions that are associated with ertugliflozin include: hypotension, diabetic ketoacidosis, intravascular volume contraction/depletion, acute kidney injury/renal impairment, lower limb amputation, hypoglycemia, and increase in low-density lipoprotein cholesterol (LDL) [5]. Renal function should be assessed before initiation and periodically with use of ertugliflozin [5]. Ertugliflozin should be renally adjusted according to the dosing schedule in Table 1.
Conclusion
This new once-daily SGLT2 inhibitor, ertugliflozin, will add to the competition of the class. However, it could serve as an additional option for better glycemic control amongst individuals with T2DM while also reducing body weight and blood pressure which could benefit the individual's overall health.
For more articles in Open Access Journal of Case Studies please click on: https://juniperpublishers.com/jojcs/index.php



No comments:
Post a Comment