Juniper Publishers- Open Access Journal of Case Studies
First Trimester Medical Pregnancy Termination: When Bleeding and Pain are not Caused by Expulsion. A Case Report of Uterine Rupture
Authored by Matthey Page C
Abstract
Introduction: Uterine rupture is a rare complication of misoprostol when used to induce first trimester medical termination of pregnancy.
Case: A 35-year-old patient gravida 5, para 3, known for a scarred uterus and hospitalised for onsite first trimester termination at 8 weeks and 2 days of pregnancy. After receiving her first dose of 400mcg of oral misoprostol, the patient developed acute abdominal pain associated with heavy vaginal bleeding. Vaginal ultrasound showed the retention of the pregnancy without sign of uterine rupture. A second dose of 400mcg of oral misoprostol was administered. We noticed persistent moderate vaginal bleeding and moderate abdominal pain without expulsion. We then proceeded to remove the pregnancy surgically. Following dilatation and curettage, a perforation of the anterior uterine wall was suspected. During laparoscopy, the complete rupture of the uterine scar covered with peritoneum was diagnosed.
Conclusion: Uterine rupture is a rare complication of misoprostol use, especially during first trimester pregnancy termination. We suggest a low threshold of suspicion in patients presenting abdominal pain with a known scarred uterus.
Keywords: First trimester medical pregnancy termination; Misoprostol; Uterine rupture
Introduction
Misoprostol is used off label in gynecology to induce uterine contractions. Its use in association with mifepristone is considered to be a safe method for pregnancy termination in the first trimester [1].
Most frequent side effects of misoprostol involve the digestive system (abdominal pain, diarrhea) or fever [2]. Uterine rupture has also been described in the literature in association with the use of misoprostol [3]. The incidence is low. The risk is known to increase throughout the pregnancy and in scarred uterus. Nevertheless, seldom cases of uterine rupture have been reported after the use of misoprostol in the first trimester. Here, we report the case of a patient, known for 3 prior caesareans, who suffered a uterine rupture after the use of misoprostol, even though the pregnancy was described by ultrasound to be located away from the uterine scar.
Case
A 35-year-old patient, gravida 5, para 3, with three prior C-sections underwent a first trimester medical termination at 8 weeks and 2 days. The patient was known for a prior first trimester medical termination, first caesarean for fetal distress more than 6 years ago, a second caesarean in an emergency setting for uterine rupture and 2 years ago a third elective caesarean with an asymptomatic dehiscence of the uterine scar discovered during the surgery. The intra-uterine localisation was confirmed by vaginal ultrasound. The lower uterine segment was not described Figure 1.
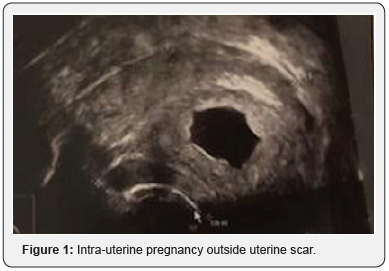
Following our hospital protocol and in accordance to international guidelines [4], we proceeded with delivering one dose of Mifepristone 200mg followed two days later by two doses of 400mcg of oral misoprostol at 3 hours interval. The patient entered our hospital for the first dose of misoprostol with a hemoglobin of 111G/l. She received the first dose of misoprostol in association with 500mg paracetamol, 30mg of Codeine and 600mg of Ibuprofen. One hour later, she suffered from acute abdominal pain (pain scale 9/10) associated with heavy vaginal bleeding (300ml in total). No change in hemodynamic parameters was reported. A uterine rupture was suspected. An emergency vaginal ultrasound showed the retention of the pregnancy without sign of intra-abdominal bleeding. A control blood count showed a stable hemoglobin of 109G/l. The pain and the bleeding spontaneously decreased (pain scale 5/10). The favorable development without medical intervention led us to decide for a stationary observation in the hospital.
After 3 hours of expectant management, without any new reappearance of acute pain, we administered a second dose of 400mcg of misoprostol. After the second dose, persistent moderate bleeding associated with moderate abdominal pain was observed, without resurgence of acute pain, for which we suggested an oversight in the hospital. At this time, the total blood loss was estimated to be 450ml.
Constant pain (5/10), increasing bleeding, associated with a drop of hemoglobin up to 73G/L motivated the decision of an emergency surgical removal of the pregnancy. A repeated ultrasound before the surgery showed the persistence of the pregnancy without any sign of intra-abdominal fluid.
In the operating room, we confirmed moderate to heavy vaginal bleeding. The cervix dilatation was spontaneously evaluated ad Hegar 10. The aspiration of the pregnancy was uncomplicated. The introduction of a curette lead to the suspicion of an anterior uterine wall dehiscence. We converted the surgery to laparoscopy. As we entered the camera in the abdomen, there was no sign of uterine rupture. Only a small amount of blood in the vesico-uterine pouch was visible.
A curette was inserted again intra-vaginaly which allowed us to see a covered perforation of the uterine wall by the peritoneum. The incision of the peritoneum revealed the complete rupture of the uterine scar Figure 2.
Cystoscopy was undertaken to exclude an extension of the tear to the bladder. The rupture was sutured during the laparoscopy procedure Figure 3.
In the post-operative setting, the patient was transfused with 2 units of blood before being discharged home the next day. An ultrasound two months later confirmed a healing scar Figure 4.
Discussion
The clinical symptoms associated with a complete rupture of the uterine scar, suggest that the uterine rupture took place after the first dose of misoprostol. This hypothesis explains the persistent pain presented by the patient and the absence of expulsion (no uterine contraction possible). Uterine rupture can present itself with intra-abdominal heavy bleeding. As demonstrated in this case, only moderate signs such as constant vaginal bleeding associated with one acute episode of abdominal pain and the absence of expulsion can also be the only symptoms present.
Misoprostol is considered safe during first trimester termination of pregnancy. Nevertheless, this case is a good reminder that a medical pregnancy termination should never be undertaken lightly. First and foremost, intra-uterine pregnancy needs to be confirmed. For patient who underwent a previous caesarean, the location of the pregnancy and trophoblast away from the uterine scar must be asserted. Ultrasonographic criteria for a definitive diagnostic have been postulated in different studies. The presence of a healthy myometrium between the bladder and the gestational sac must be established [5]. For patients who underwent multiple anterior caesarean, to our knowledge, there is no study showing a threshold in size for the inferior uterine wall. It is debatable whether the risk of misoprostol use is greater than a surgical aspiration for these patients.
When uterine dehiscence or rupture is suspected in a stable patient, ultrasonography may be a useful tool to confirm the diagnosis [6]. As shown in our patient, the ultrasonography can also be misleading, showing no sign of intra-abdominal fluid and falsy suggesting that the pain experienced by the patient was connected to uterine contractions and not rupture.
The recommended dose of misoprostol in first trimester pregnancy termination does not differ depending on the route of administration. Similarly, there is no required adaptation necessary for patients with previous caesarean under 26 weeks of gestation [7]. The recommended dose for misoprostol in first trimester termination in the new FIGO guidelines is 800mcg sublingual, per vaginal or buccal every 3 hours for 2 to 3 doses. Our hospital protocol delivers two doses of 400mcg of misoprostol. The symptoms suggest that the rupture occurred already after the first dose. This fact suggests that the risk of rupture could be better linked to the use of misoprostol than the actual dosage. Uterine rupture has also been described in the literature after the use of misoprostol in unscarred uterus [3].
It is therefore critical before any pregnancy termination in the first trimester to evaluate the risk of uterine rupture for each patient (previous caesarean, number of anterior uterine surgery, uterine wall disease). Most of all, this case is a good reminder that even though the risk of uterine rupture is low, it is inherent to the prescription of drugs inducing contractions such as misoprostol.
Conclusion
Uterine rupture is a rare complication associated with the use of misoprostol in first trimester pregnancy termination. Nevertheless, it is necessary to have a low suspicion threshold for patient with a scarred uterus. There is no consensus as to whether it is necessary to measure the inferior uterine wall for patient with prior caesarean who undergo a first trimester pregnancy termination. We suggest that these patients should receive medical abortion in the hospital setting or a surgical removal of the pregnancy under ultrasound.
To know more about Juniper Publishers please click on: https://juniperpublishers.com/manuscript-guidelines.php
For more articles in Open Access Journal of Case Studies please click on: https://juniperpublishers.com/jojcs/index.php



No comments:
Post a Comment