Juniper Publishers- Open Access Journal of Case Studies
Role of Monoclonal Antibodies in the Treatment of COVID-19- A Review Article
Authored by Rajinder Pal Singh Bajwa
Review
COVID-19 is caused by a coronavirus designated as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). First reported cases were a cluster of pneumonia patients in Wuhan, China in December 2019. Since then, the SARS-CoV-2 virus has spread worldwide. World Health Organization declared COVID-19 a global pandemic on March 11, 2020. As of May 6, 2021, there has been154,815,600 confirmed cases of COVID-19 with 3,236,104 deaths across the globe[1]. In United States, the disease burden is 32,313,016 with 575,491 deaths. The SARS-CoV-2 virus is causing havoc in multiple communities/countries, it has changed the way we live and is testing the limits of already strained Healthcare Systems across the world. Ongoing COVID-19 pandemic will easily go in the history books as the deadliest pandemic in the modern world history. This article intends to describe role of various monoclonal antibodies available for the treatment of COVID-19.
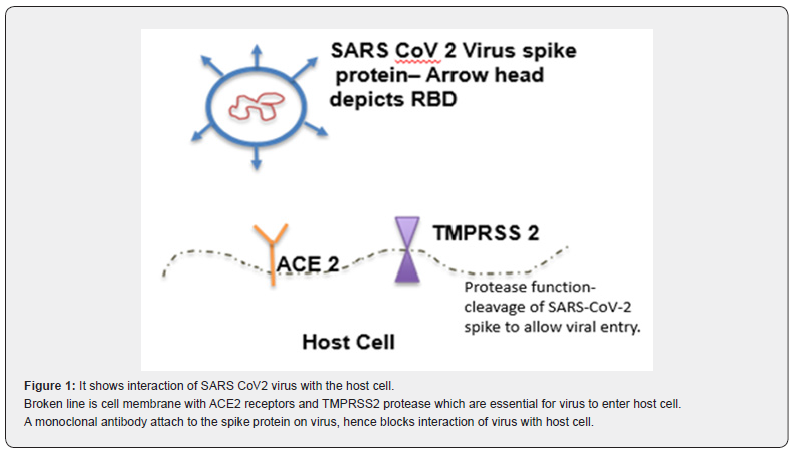
SARS-CoV-2 is an enveloped positive stranded RNA virus. It belongs to Beta coronavirus genus. Coronaviruses as a group, have largest and most complex genome of all RNA viruses. The viral genome encodes for both structural and nonstructural proteins [2]. SARS-CoV-2 virus has a spike (S) protein, which projects through the viral envelope. S protein is a glycoprotein that forms homotrimers and is one of the most important structure which helps SARS-CoV-2 virus to enter host cells.
Patient is usually infected by inhaling SARS-CoV-2 viral particles. On entering the airways, virus particles encounter respiratory epithelial cells. The SARS-CoV-2 virus attaches to the Angiotensin Converting Enzyme 2 (ACE 2) receptors on the epithelial cells through receptor binding domain (RBD) on the spike protein. Once the spike protein attaches to the ACE 2 receptors it cannot open, close or bend freely, locking it in place. Transmembrane protease, serine 2 (TMPRSS 2) on the host epithelial cells cut the spike protein in the specific locations [3]. These cleaved portions of spike protein fall away, exposing the previously hidden parts of the spike protein. This cleaved spike protein now undergoes series of conformational changes, attaching to host cell and ultimately fusing with the host cell and injecting virus genome into the cell. Once virus genome is in the host cell, its highjacks the host machinery to replicate and cause further damage. This attachment process has been used to create a novel treatment options like neutralizing monoclonal antibodies and vaccines (Figure 1).
Clinical spectrum of SARS-COV-2 infection ranges from asymptomatic carriers to patients with critical illness requiring ventilator support. Approximately 80% of patients infected with SARS-CoV-2 will have mild-to-moderate self-limiting symptoms. Around 15% will progress to severe disease requiring hospitalization and less than 5% cases will develop critical illness needing mechanical ventilation [4]. National institutes of Health (NIH) have classified illness into following categories, which help in defining treatment, gives uniformity of definition for research purposes and may have some prognostic value [5].
a) Asymptomatic or Presymptomatic Infection: Patients with positive SARS-CoV-2 test (PCR or an antigen test) but without any symptoms.
b) Mild Illness: Patients with milder signs and symptoms of COVID-19 like fever, cough, myalgias, loss of taste and smell etc., who do not have shortness of breath, or abnormal chest x-ray or CT.
c) Moderate Illness: Patients who show evidence of lower respiratory disease on clinical exam or imaging and have an oxygen saturation (SpO2) ≥94% on room air.
d) Severe Illness: Patients with SpO2 less than 94% on room air, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) <300mm Hg, respiratory rate >30 breaths/min, or lung infiltrates on imaging more than 50%.
e) Critical Illness: Patients with respiratory failure, septic shock, and/or multiple organ dysfunction.
It is difficult to predict who will progress to critical illness, but generally speaking patients with advanced age and underlying risk factors like diabetes mellitus, chronic kidney disease, obesity with BMI > 35 and immunosuppressed host are more likely to progress to severe illness as shown in the Table 1.

Treatment of COVID-19 is rapidly evolving. Multiple clinical trials are ongoing and literature on treatment modalities of COVID-19 is changing at light speed. So far only one medication (Remdesivir) has received full FDA approval for treatment. Other medications/therapies are being used under emergency use authorization or off-label. Here we will only discuss role of monoclonal antibodies in the treatment of COVID-19 and will briefly present evidence in support of these therapies.
Based on our current knowledge of pathogenesis of COVID-19, we know that antiviral medications like Remdesivir and neutralizing antibodies will work best in early stages of the disease. In later stages, it is the inflammatory response which is causing damage, hence treatments which modify inflammation are used, like dexamethasone and tocilizumab.
Neutralizing monoclonal antibodies against spike protein
Bamlanivimab 700mg + Etesevimab 1400mg
Casirivimab 1200mg + Imdevimab 1200mg
a) These monoclonal antibodies neutralize the SARS-CoV-2 virus by attaching to receptor binding domain of SARS-CoV-2 spike protein, thus blocking attachment of SARS-CoV-2 virus to the host ACE2 receptors.
b) Have received emergency use authorization through FDA for treatment of mild to moderate COVID-19 patients with underlying risk factors (see table 1) for progression to severe disease.
c) Only to be used in the ambulatory settings and not for patients hospitalized with COVID-19.
d) Treatment is most effective if started within 10 days of being symptomatic.
On November 9, 2020 the FDA issued an Emergency use authorization for a single infusion of 700mg Bamlanivimab for the treatment of mild to moderate COVID-19 in adult and certain pediatric patients. Soon thereafter on November 21, 2020 combination of monoclonal antibodies Casirivimab and Imedevimab was granted emergency use authorization for similar indications. On February 9, 2021 another combination monoclonal antibody Bamlanivimab and Etesevimab received emergency use authorization. Owing to growing concern of certain SARS-CoV-2 variants not effectively being neutralized by Bamlanivimab in lab studies, FDA revoked emergency use authorization for monoclonal antibody therapy Bamlanivimab when administered alone on April 16, 2021.
At the time of writing of this manuscript, two combination monoclonal antibodies are being used in United States under emergency use authorization for treatment of mild to moderate COVID-19 infection in at risk patients. These monoclonal antibodies are given as 1-time intravenous infusion. Treatment with monoclonal antibodies does not change isolation requirements and patients are asked to continue home isolation for time period defined by the health department. Also given long half-lives of monoclonal antibodies, it is currently suggested that these patients should not receive COVID-19 vaccine for up to 90 days after the infusion.
Clinical evidence for use of Bamlanivimab and Etesevimab comes from BLAZE-1 trial [6]. In this trial, patients with mild to moderate COVID-19 infection with underlying risk factors were given either combination of Bamlanivimab + Etesevimab or placebo within 3 days of positive test. End point for the study was COVID-19 related hospitalizations or any cause death by day 29. In treatment group out of 518 patients, 11 had COVID-19 related hospitalization and no deaths. In placebo group 36 defined events happened out of 517 participants. These 36 defined events in placebo group include 10 deaths. There was 70% relatively risk reduction in COVID-19 related hospitalizations or any cause death by day 29 in patients who received Bamlanivimab and Etesevimab versus placebo.
Clinical evidence for use of Casirivimab and Imedevimab comes from Phase 3 results from the R10933-10987-COV-2067 study off patients with mild to moderate COVID-19 infection with underlying risk factors [7]. This trial compared 1,355 participants who received Casirivimab 1,200 mg plus Imdevimab 1,200 mg to 1,341 participants who received placebo. Endpoint of this study was COVID-19 related hospitalizations or death through day 29. Out of 1355 patients receiving Casirivimab and Imedevimab, 18 had either hospitalization or death through day 29. In placebo group, out of 1341 patients, 62 participants either had COVID-19 related hospitalizations or death through day 29. This study showed 71% relative risk reduction in COVID-19 related hospitalization or death through day 29 in patients who received Casirivimab and Imedevimab compared to placebo.
These studies show almost similar efficacy of 2 monoclonal antibody combos in preventing hospitalization or death in patients with mild to moderate COVID-19 infection in at risk patient. These monoclonal antibodies should be used when available for appropriate patients. However, there is a constant concern that these monoclonal antibodies may not help if there is significant change in the spike protein structure because of mutations in the viral genome. Constant surveillance needs to be done to identify the types and frequencies of circulating SARS-CoV-2 variants along with susceptibility of different variants to available neutralizing antibodies.
Antibodies regulating immune microenvironment
No monoclonal antibody has received emergency use authorization or full approval for treatment of COVID-19. Multiple agents in this class are being evaluated in clinical trials. There has been emerging data for use of tocilizumab in serious COVID-19 infections.
Tocilizumab
It is a recombinant humanized anti-IL 6 receptor monoclonal antibody. IL-6 is secreted by macrophages in response to various inflammatory stimuli and mediates a variety of immunological responses. Tocilizumab blocks the IL-6 receptors, which leads to a reduction in cytokine and acute phase reactant production. It is not yet approved or has been authorized for emergency use for COVID-19 treatment. Since inflammatory cytokines play a significant role in rapid deterioration of patients, medications like IL-6 inhibitors and steroids may help these patients. In critically ill adult patients with COVID-19 receiving organ support in ICU and/or CRP >75, treatment with the IL-6 receptor antagonists, improved outcomes including survival as shown in REMAP-CAP and RECOVERY studies [8,9]. Current NIH COVID-19 treatment guidelines recommend use of tocilizumab along with dexamethasone in hospitalized patient requiring oxygen delivery through a high-flow device or noninvasive ventilation and in patients who require invasive mechanical ventilation or ECMO.
Tocilizumab should not be used in patients with deranged liver functions and in patients with platelet count <50/mm3. Use caution in patients with diverticulitis due to risk of GI perforation. Active bacterial, mycobacterial, fungal and protozoal infections must be ruled out before starting tocilizumab, as it may lead to worsening of underlying infection and serious complications.
Conclusion
In summary, treatment of COVID-19 is changing rapidly and more and more therapeutics are being studied in clinical trials. Monoclonal antibodies are important members of therapeutics against COVID-19 and are finding place in treatment guidelines. These drugs should be used when available in appropriate setting.
To know more about Juniper Publishers please click on: https://juniperpublishers.com/manuscript-guidelines.php
For more articles in Open Access Journal of Case Studies please click on: https://juniperpublishers.com/jojcs/index.php



No comments:
Post a Comment