Juniper Publishers- Open Access Journal of Case Studies
The Warburg Effect - An Onco-hematologic Emergency?
Authored by Poggi Guido
Abstract
Warburg effect is a rare and potentially life-threatening metabolic complication occurring in oncologic patients. The Authors report the case of a 79-year-old man affected by diffuse large B-cell lymphoma presenting with severe lactic acidosis and hypoglycaemia both of which were refractory to conventional management and they critically review the available literature.
Keywords:Warburg effect; Lactic acidosis; Hypoglycaemia; Hematologic malignancies; Cancer cells; Chronic hepatitis C; Nephrology; Bladder tumour
Introduction
The combination of lactatemia and severe untreatable hypoglycaemia is a rare metabolic complication of hematologic malignancies. It occurs more frequently in patients affected by lymphoma, and it is generally associated with an ominous prognosis. It is secondary to an avid consumption of glucose by the cancer cells that even under aerobic conditions switch their glucose metabolism from the oxidative pathway to the glycolytic pathway, leading to increased lactate production. This metabolic shift is known as the “Warburg effect”. Here we will discuss a patient with diffuse large B-cell lymphoma (DLBCL), who presented with hypoglycaemia and lactic acidosis, and we will review similar cases from the relevant literature, which we believe warrant the consideration of the Warburg effect as an onco-hematological emergency.
Case Description
A 79-year-old man was referred to our ward for evaluation of massive hepatomegaly discovered two weeks earlier, when he presented to the emergency department complaining of weight loss, nausea, vomiting and abdominal discomfort. In the emergency department, he was diagnosed with an acute renal injury of prerenal origin, presumptive subacute cholangitis and chronic hepatitis C. He was transferred for evaluation in the nephrology department and received treatment with fluid replacement, resulting in partial improvement of renal function. He was subsequently discharged and referred to us to complete the diagnostic workup. At the time of admission, his therapeutic regimen included edoxaban and flecainide, as well as amlodipine and losartan for arterial hypertension. His medical history was notable for tuberculous spondylodiscitis at age 20, and a cholecystectomy at age 46. In 2017, two years before he was referred to us, he was diagnosed with transitional cell bladder carcinoma that did not infiltrate the muscular layer and was treated with transurethral resection of a bladder tumour (TURBT) followed by intravesical chemotherapy. He was also diagnosed with atrial fibrillation and treated with direct-acting oral anticoagulants (DOAC), and in 2018 he required electric cardioversion. Familial history was notable for the untimely death of his mother at age 35 for a hepatic tumour, and the death of his brother at the age of 66, for multiple myeloma.
Upon arriving at our ward, the patient was alert and presenting no noticeable neurological alterations. He appeared unfit, complained of profound fatigue, anorexia and abdominal discomfort. He reported to have lost approximately 10kg in the previous two months and denied any episodes of fever or drenching sweats. His temperature was 36.8 °C, the heart rate was 98 beats per minute, the respiration rate was 18 breaths per minute, and the oxygen saturation was at 97% while breathing ambient air. Physical examination revealed massive hepatomegaly, extending on the lower margin to 2cm below the transverse umbilical line, and concomitant splenomegaly with a palpable lower pole 4cm below the costal arch. The ultrasound confirmed massive hepatosplenomegaly; however, the hepatic parenchyma revealed no nodules, but only a focal solitary hypoechoic area of non-nodular appearance. A contrast-enhanced total body CT scan was performed and confirmed the ultrasound findings with liver and splenic homogeneous enlargement and further revealed the involvement of multiple retroperitoneal lymphnodes not found at the US scan. A complete blood count, including a metabolic panel and arterial blood gas analysis, was performed (Table 1-2), revealing significant hypoglycaemia and metabolic acidosis with a high anion gap, consistent with lactic acidosis.
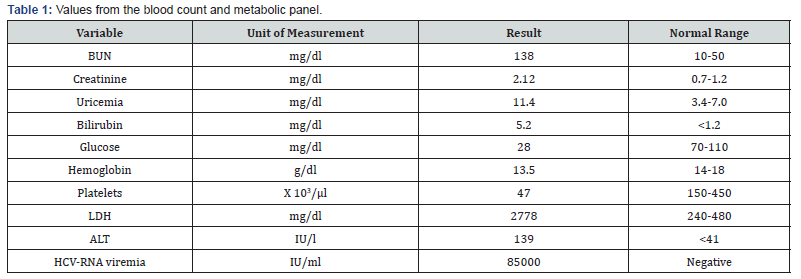

Despite the severe hypoglycaemia, the patient was not complaining of neuroglycopenic symptoms. Endocrinological investigation showed that the adrenal and thyroid functions were normal. Other markers, including insulin, pro-insulin and C-peptide, were all reduced, excluding both endogenous insulin production and exogenous administration as a cause for hypoglycaemia. Biopsies of the bone marrow and liver revealed a massive infiltration of diffuse large B-cell lymphoma (DLBCL), expressing CD 20+, CD 30+ and EBV+ with high proliferative activity (Ki-67 = 90%). Correction of the hypoglycaemia with a continuous IV infusion of a 10% glucose solution and thiamine failed and exacerbated the lactatemia. Therefore, since the patient did not present hypoglycaemic symptoms, the attempt to correct the hypoglycaemia was discontinued. Unfortunately, the rapid progression of thrombocytopenia and the progressive increase in creatinine levels excluded chemotherapy as a therapeutic option. The patient, therefore, received treatment with prednisone (60mg/day) and a weekly regimen of rituximab, which led to a gradual improvement of both the clinical presentation and the blood parameters over the first three administrations of rituximab. However, shortly after that, the clinical picture rapidly worsened, and the patient died.
Discussion
The clinical presentation of Non-Hodgkin’s Lymphoma (NHL) varies depending on the subtype and the involved sites, with symptoms including enlarged palpable lymphadenopathy, B-symptoms (fever, weight loss, night sweats), and symptoms secondary to compression of adjacent structures. In rare cases, the clinical presentation may include metabolic complications [1-3] such as lactic acidosis and hypoglycemia. Lactate is a by-product of glucose metabolism, under anaerobic conditions, when pyruvate is reduced to lactate by lactate dehydrogenase (LDH). In normal conditions, most of the lactic acid is cleared by the liver (80– 90%) and converted to glucose through gluconeogenesis (Cori’s cycle), while the remainder is secreted by the kidneys [4]. Lactate accumulation leads to lactic acidosis when the concentration of lactate in whole blood exceeds 5mmol/L with a pH below 7.35 [3]. Lactic acidosis can result either from hypoperfusion (type A) or from overproduction or decreased clearance of lactate (type B). Type A lactic acidosis can result from clinical settings of hypoxia and inadequate tissue perfusion due to septic or hypovolemic shock. Type B, however, occurs in normal perfusion states and is associated with malignancies, underlying liver or kidney failure, diabetes mellitus, thiamine deficiency, drugs and toxins (e.g., alcohols, metformin, salicylates, reverse transcriptase inhibitors, cyanides) and hereditary enzymatic defects [5,6]. In the case of malignancies, as observed by Otto Warburg many years ago, cancer cells consume glucose and excrete lactate at a significantly higher rate compared to healthy cells, even in normoxic conditions [7]. The increased rate of glycolysis is due to the aberrant expression or over-expression of glycolytic enzymes, as part of the malignant process. One such example is hexokinase II, a rate-limiting enzyme involved in glycolysis, whose activity is regulated by the IGF signalling pathway, which is often defective in malignancies [8,9]. However, even though cancer cells produce increased amounts of lactate, lactic acidosis does not develop until the measure of lactate exceeds the limit of hepatic clearance and overwhelms the renal clearance, which could happen when the underlying disease has developed multiple metastases or diffused infiltration of the liver [10], as it is in the majority of reported cases of leukaemia and lymphoma and in the case we report here.
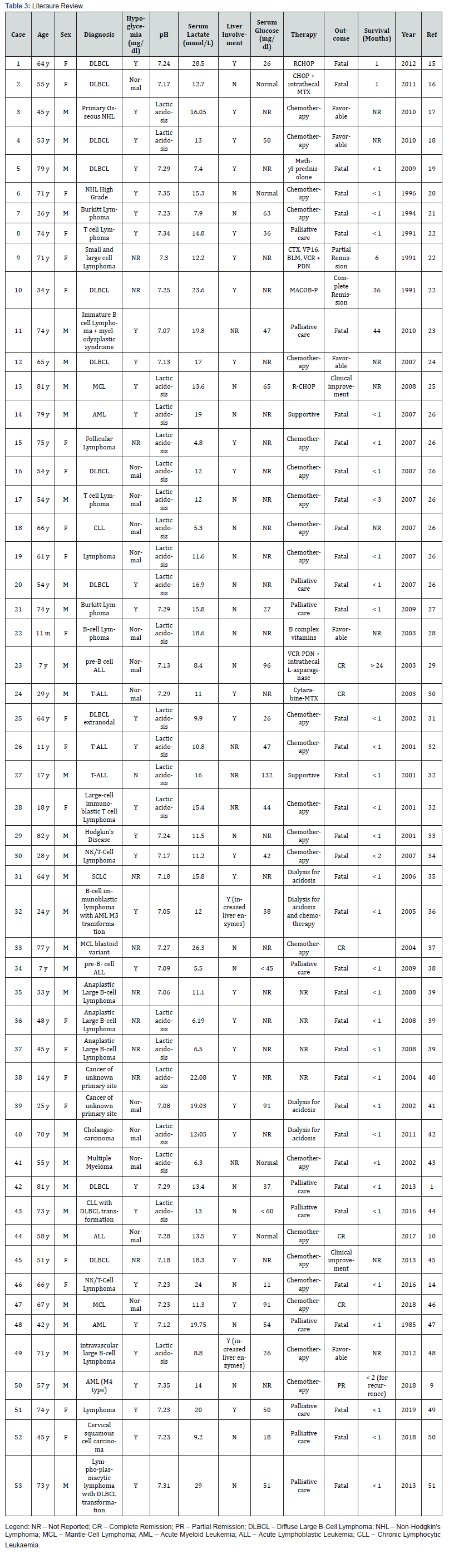
A literary review of 53 relevant clinical cases published between the years 1985-2019, focused on cases of haematological malignancies associated with lactic acidosis, has shown that as a consequence of the increased glucose consumption by the tumour, 29 (54.7%) of these patients were in a state of persistent hypoglycaemia and in 17 cases (32.1%) there was no report of neurologic deficiencies. This peculiarity is due to an ability of the brain to employ lactate as a primary source of energy, providing the brain with a protective mechanism from systemic hypoglycaemia. However, the mechanism by which this phenomenon occurs remains undetermined [11-13]. Among the 53 reviewed cases, 26 cases reported liver involvement in the development of the pathology, out of which 69.2% of the cases resulted in fatalities. It is relevant to note that the other 30.8% of cases (with reported favourable outcomes) have all engaged in a protocol of chemotherapy. The unusual clinical presentation that we are reporting, characterised by severe, untreatable hypoglycaemia, lactic acidosis, and liver involvement, was only described in 11 malignancy cases, all are cases of lymphoma, and predicts a very poor prognosis (Table 3).
Presumably, the poor prognoses are due to the aggressive nature of the disease, the fact that these cases are often diagnosed at a rather late stage in the disease progression, and indeed, any part of the clinical presentation may be justifiably suspected as a more prevalent condition. However, it is precisely because of the rarity and peculiarity of this clinical presentation that we believe the consideration and exclusion of a potential hematological emergency should be prioritised in the clinical approach. The Warburg effect, stemming from the alteration of cancer metabolism, seem to be manipulable using dichloroacetate (DCA), which is not yet indicated for the treatment of cancer but is used to treat lactic acidosis and diabetes. Despite the lack of official indication for DCA in this setting, there is an accumulating body of evidence to support its efficacy. DCA is thought to mitigate the Warburg effect by inhibiting pyruvate dehydrogenase kinase, leading to the activation of pyruvate dehydrogenase, and by that process DCA is able to stimulate cellular respiration through oxidative phosphorylation, rather than strict glycolysis, offsetting the relative metabolic advantages that neoplastic tissues hold over their surrounding healthy tissue. Moreover, due to the activation of mitochondrial respiration, DCA can limit the amount of lactate produced by the tumour, changing its chemical environment and inhibiting its cellular proliferation, as well as restoring a degree of apoptotic function through the increase in cytochrome c [52].
However, although DCA represents a promising prospect, it is important to note that the encouragement of mitochondrial activity could have deleterious consequences for the nervous system, as these tissues rely primarily on glycolysis and may lack the cellular functions to sustain the increase in free radicals. Nevertheless, in the context of Warburgism, where the severe systemic hypoglycaemia does not result in neuroglycopenic symptoms, it seems reasonable to hypothesise that the cells of the nervous system have already gone through the necessary remodeling to endure this metabolic shift. However, we are currently unaware of such research.
To know more about Juniper Publishers please click on: https://juniperpublishers.com/manuscript-guidelines.php
For more articles in Open Access Journal of Case Studies please click on: https://juniperpublishers.com/jojcs/index.php



No comments:
Post a Comment